Stockholm Bioinformatics Center, SBC
Lecture notes: Structural biochemistry and bioinformatics 2001
Lecture 30 Nov 2001,
Per Kraulis
3. Network properties
Metabolic and signalling networks are complex: There are many
nodes (proteins, particles, molecules) and many connections
(interactions). This is obvious from nearly any illustration
of a network. And even if one can define sub-networks that can be
meaningfully described in relative isolation (such as the citric acid
cycle, or the ras p21 signalling network), there are always
connections from it to other networks.
The overall structure of a network can be described by several
different parameters. For example, the average number of
connections a node has in a network, or the
probability that a node has a given number of
connections. Theoretical work has shown that different models
for how a network has been created will give different values for
these parameters.
One can also plot the relative frequency of each node
as a function of the number of connections of the
nodes. Both axes in the plot uses logarithmic scales. Using data from
real networks or from simulations of different types of randomized
networks, one obtains log-log plots that turn out to have
characterisic shapes depending on how the properties of the networks
and how they were generated. Examples are shown in the figures below.
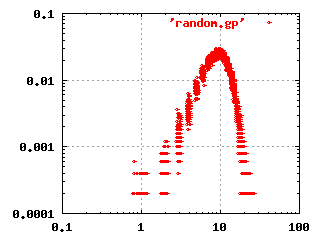 The classical random network theory by Erdös and
Renyi (1960) states that given a set of nodes, the
connections are made randomly between the nodes. This gives a network
where most nodes have the same number of connections. The log-log
frequency plot for this kind of network is shown to the left.
The classical random network theory by Erdös and
Renyi (1960) states that given a set of nodes, the
connections are made randomly between the nodes. This gives a network
where most nodes have the same number of connections. The log-log
frequency plot for this kind of network is shown to the left.
Recent research has shown that this model does not fit the structure
found in several important networks, such as the Internet, World-Wide
Web, power grids, or social networks. Instead, these complex networks
are better described by a so-called scale-free, hierarchical
model where most nodes have only a few connections, but a few
nodes (called hubs) have a very large number of connections.
Recent work by Jeong, Barabasi et al (Nature (2000) 407,
651-654), indicate that metabolic networks are examples of
such scale-free networks. This result is important, and will
probably lead to new insights into the function of metabolic and
signalling networks, and into the evolutionary history of the
networks.
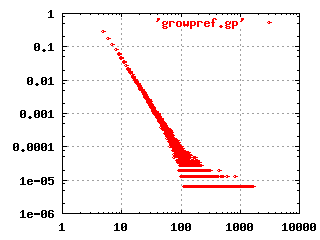 The image to the left shows the characteristic shape of a
scale-free, hierarchical network in a log-log plot. This
particular plot was produced by simulating a growing network, where
each new node is connected randomly to existing nodes, but with a
higher preference for already highly connected nodes.
The image to the left shows the characteristic shape of a
scale-free, hierarchical network in a log-log plot. This
particular plot was produced by simulating a growing network, where
each new node is connected randomly to existing nodes, but with a
higher preference for already highly connected nodes.
Robustness is another important property of metabolic
and signalling networks. This is the ability of the network to produce
essentially the same behaviour even when the various parameters
controlling its components vary within considerable ranges. For
instance, recent work (von Dassow et al, Nature (2000) 406,
188-192) indicates the segment polarity network in the
Drosophila embryo can function satisfactorily with a
surprisingly large number of randomly chosen parameter sets. The
parameters do not have to be carefully tuned or optimized. This makes
biological sense: A signalling system should be tolerant with respect
to mutations or large environmental changes.
One important aspect of metabolic and signalling networks is that they
are strongly conserved in evolution. Generally, the
same roles in the metabolic networks in different organisms are
performed by homologous enzymes, with the same kind of regulatory
mechanisms. Of course, there are cases where a non-homologous protein
is involved in one organism compared to the norm in others. There are
also also cases where an entire section of a network is missing in an
organism.
Copyright © 2001
Per Kraulis
$Date: 2001/11/19 16:18:33 $
 The classical random network theory by Erdös and
Renyi (1960) states that given a set of nodes, the
connections are made randomly between the nodes. This gives a network
where most nodes have the same number of connections. The log-log
frequency plot for this kind of network is shown to the left.
The classical random network theory by Erdös and
Renyi (1960) states that given a set of nodes, the
connections are made randomly between the nodes. This gives a network
where most nodes have the same number of connections. The log-log
frequency plot for this kind of network is shown to the left.
 The image to the left shows the characteristic shape of a
scale-free, hierarchical network in a log-log plot. This
particular plot was produced by simulating a growing network, where
each new node is connected randomly to existing nodes, but with a
higher preference for already highly connected nodes.
The image to the left shows the characteristic shape of a
scale-free, hierarchical network in a log-log plot. This
particular plot was produced by simulating a growing network, where
each new node is connected randomly to existing nodes, but with a
higher preference for already highly connected nodes.